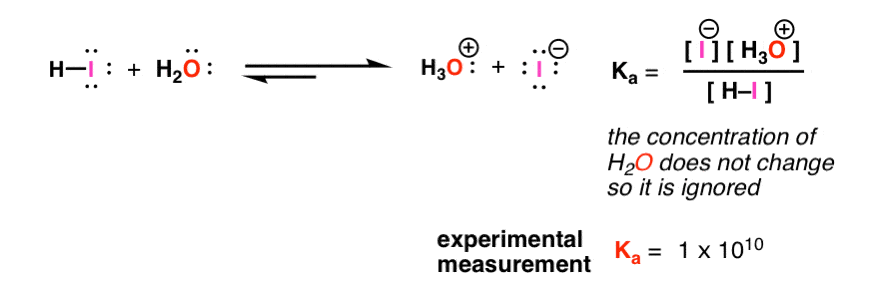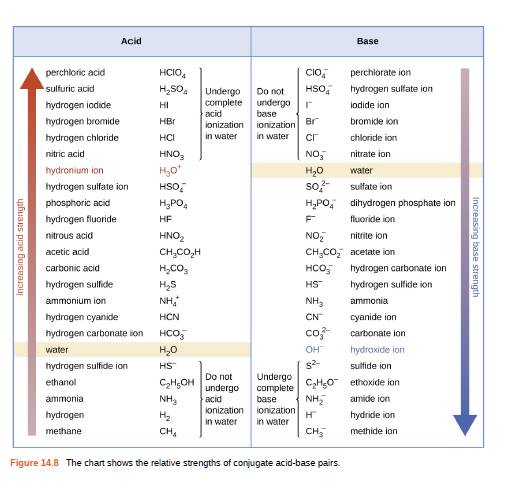
Using the K a value of 1.4 × 10 − 5 , place Al(H 2 O) 6 3 + in the correct location in Figure 14.8. | bartleby
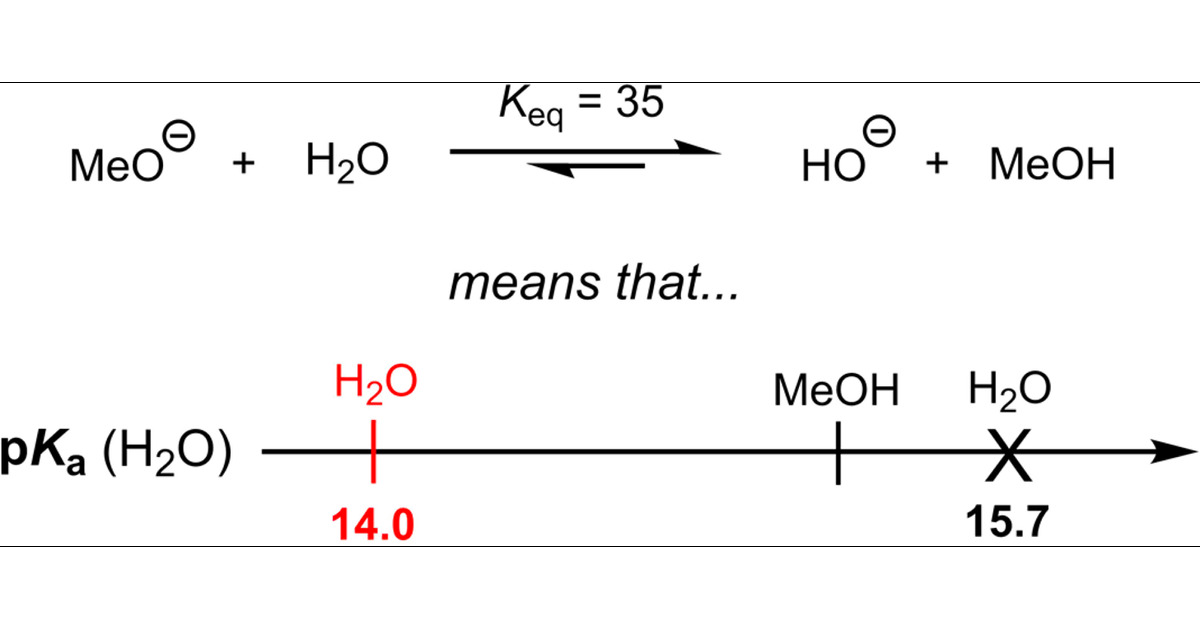
pKa Values in the Undergraduate Curriculum: What Is the Real pKa of Water? | Journal of Chemical Education
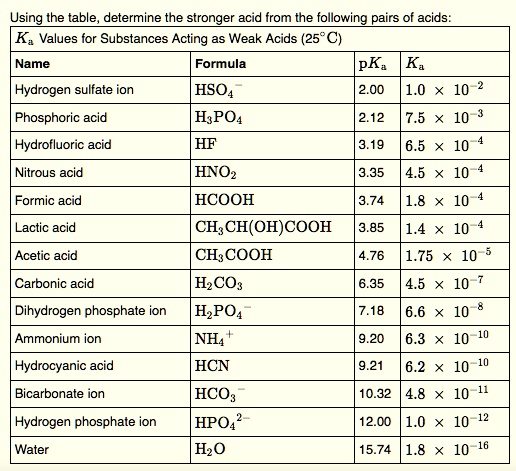
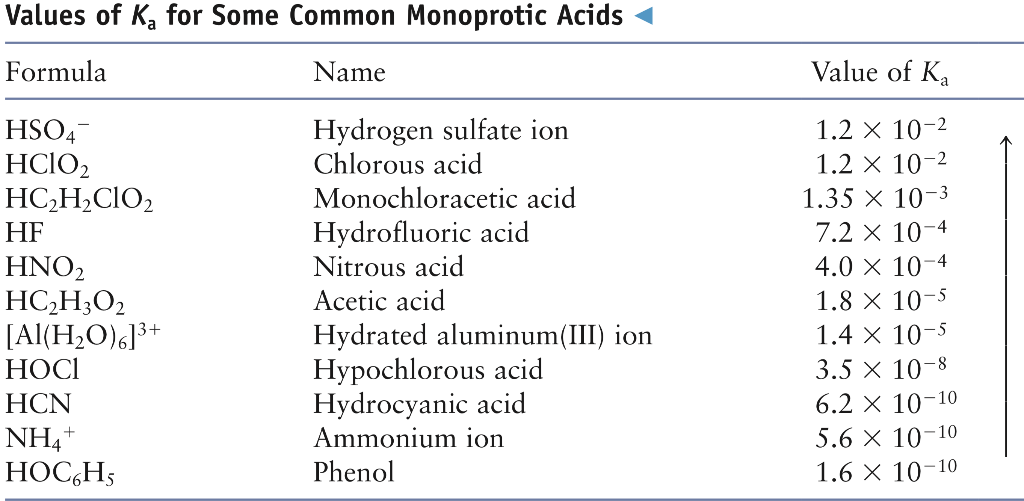
SOLVED: Using the table, determine the stronger acid from the following pairs of acids: Ka Values for Substances Acting as Weak Acids (25°C) Name Formula pKa Ka Hydrogen sulfate ion HSO4 2.00
The aluminium solution which contains [Al(H2O) 6] ^(3+) ions have the tendency to hydrolyze. Why? - Quora

Sir ncert me H2o ka SRP less than Cl- hai but overpotential ke liye Cl- prefer kiya hai Lekin class - Chemistry - - 16458413 | Meritnation.com

The value of K_(p) for the water gas reaction, CO +H_(2)O hArr CO_(2) +H_(2)is 1.06 xx 10^(5) at... - YouTube

Consider the reaction described by the chemical equation shown. C2H4(g)+H2O(l)⟶C2H5OH(l)Δ∘rxn=−44.2 kJ - brainly.com
the s†an dard enthalpy of formation of gaseous H2O at 298K is 241.82KJ/mol. Estimatr its value of 373K given the following values of the molar heat capacities at cons†an t pressure: H2O(g):35.58J/Kmol,
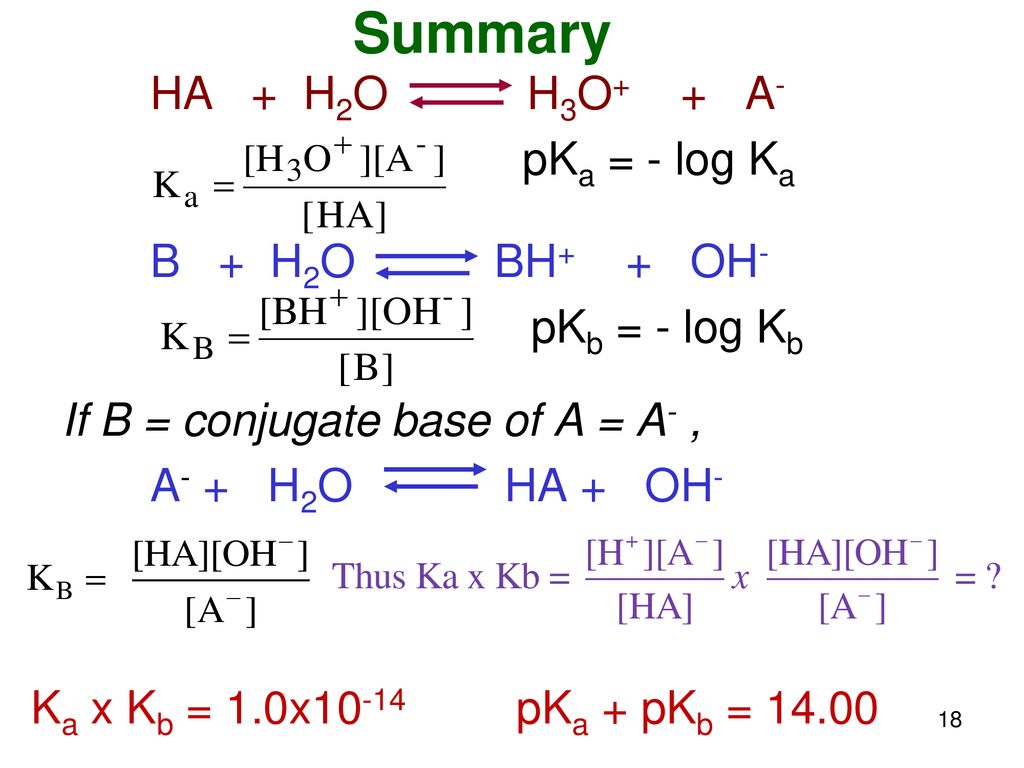
What is value of Ka and kb for water (H20) and how numerically prove that ka ×kb=kw in case of water? - Quora
Find out the Value ofequilibrium constant for the following reaction at 298 K, 2 NH3(g) + CO2 ⇌ NH2CONH2(aq) + H2O(I) - Sarthaks eConnect | Largest Online Education Community
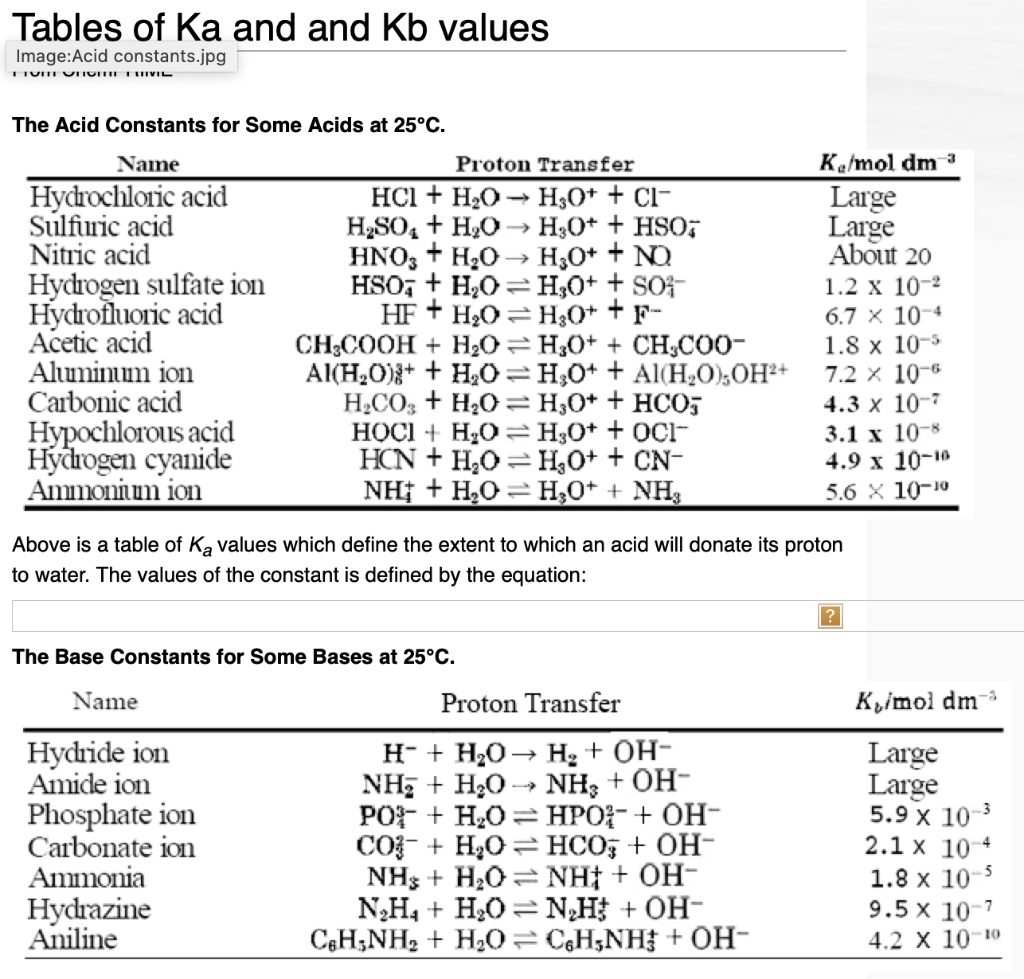
SOLVED: Tables of Ka and Kb values The Acid Constants for Some Acids at 25°C Name Proton Transfer Ka (mol dm^-3) Hydrochloric acid HCI + H2O â†' H3O+ + Cl- Large Sulfuric

OneClass: please help with part F Consider the following reaction. H2O(g) + Cl2O(f) 2 HOCI(g) K298 - ...


![ANSWERED] A buffer solution contains dissolved C6H5... - Physical Chemistry ANSWERED] A buffer solution contains dissolved C6H5... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/43165458-1658605548.8452456.jpeg)



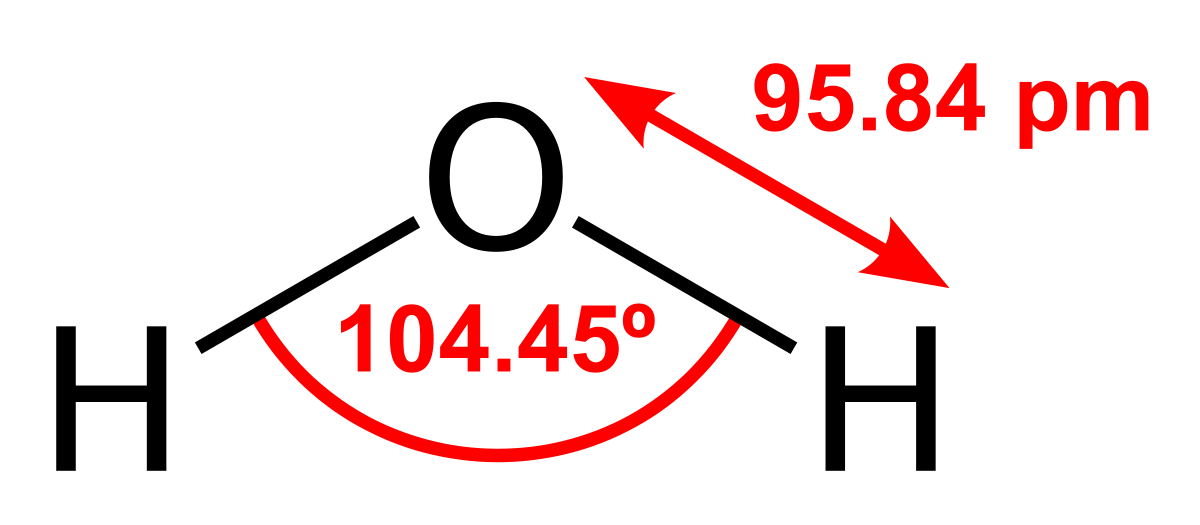

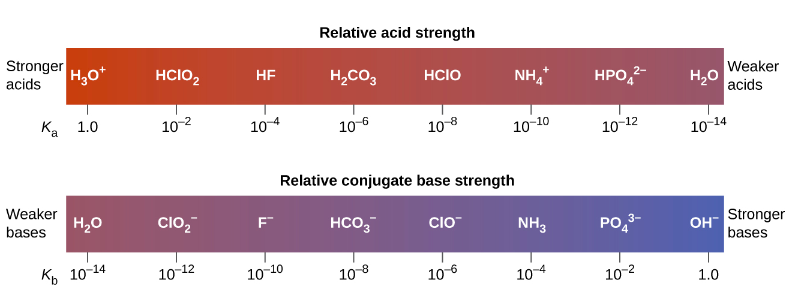


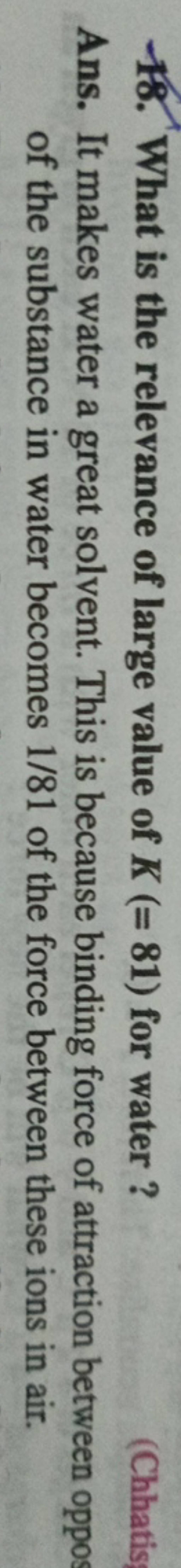
.PNG)